Brønsted-Lowry Acids and Bases
Definitions
The Brønsted-Lowry theory of acids and bases is that: acids are proton donators and bases are proton acceptors.
So therefore, in an acid-base equilibria where an acid reacts with a base, you have the proton (or H+ ion) being transfered from the acid to the base. For example.
HCl (aq) + H2O (l) ® H3O+ (aq) + Cl- (aq)
In this case the water acts as a base and accepts a proton from the HCl to form a hydroxonium ion (H3O+).
pH
The stronger an acid is, the more it dissociates (seperates when dissolved) and therefore the higher the hydrogen ion concentration. However, the concentration of hydrogen ions is often very small in a solution, so we use the following to make it into a more convenient number.
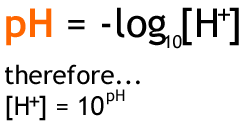
Remember that the notation [B] means: the concentration of B in moldm-3. If you haven't studied mathematics to an advanced level, you may not be familiar with logarithms. If this is the case then don't worry. All you need to be able to understand is that there is a 'log' button on all scientific calculators that will calculate this for you.
So to find the pH of a strong acid, you would simply take the concentration of this acid as the value for [H].
Ionic Product of Water Kw
Water can act as an acid or a base. And the following equilibrium exists in water:
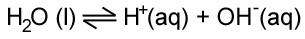
This can be expressed as an equilibrium constant using the concentrations. However, the equilibrium lies very far to the left. This means that very few hydrogen and hdroxide ions are produced and the concentration of water can be taken to be constant. Therefore we get a different expression that is called Kw.
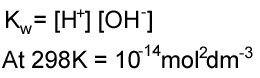
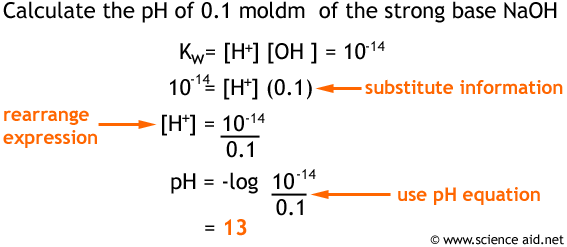
Now that we have this information it is possible to calculate the pH of strong bases, by a very simple process; have a look at the following example.
Ka and Weak Acids
As addressed above, strong acids like HCl, and bases like NaOH will dissociate (dissolve into their ions) fully. However, a weak acid will only partially dissociate in solution. Ka is known as the acid dissociation constant it is a figure that tells us by how much an acid dissociates and is calculated as follows...
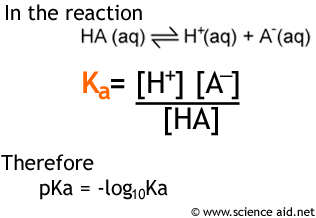
You will note that there is also an expression for pKa, this is a value that is used for the same reason as pH: it is more convenient than very small values in standard form. So to convert from pKa to Ka, you simply do 10 {to the power of} -pKa.
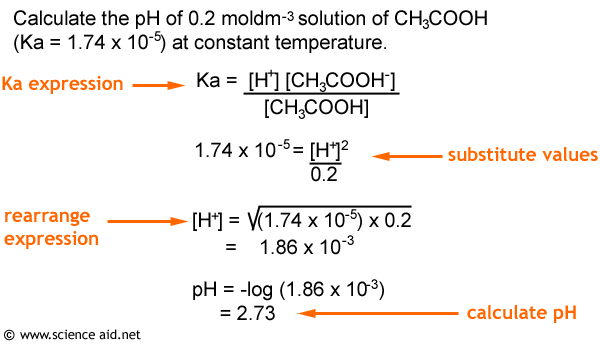
Now it is possible for you to calculate the pH of a weak acid using this information. Have a look at the worked example below.
Buffers
A buffer solution can resist changes in pH when small amounts of acid or base are added. An acidic buffer maintains a pH below 7 and consists of a weak acid and its salt. A basic buffer maintains pH above 7 and consists of a weak acid and its salt.
For example, an acidic buffer could contain ethanoic acid and sodium ethanoate. This will produce the following reactions:
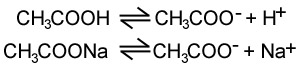
The equilibrium for the first reaction is far to the left and the equilibrium for the second reaction is pretty much 100% to the right. If an acid is added the H+ reacts with the excess CH3COO- and this become ethanoic acid, which doesn't change the equilibrium. If a base is added the OH- reacts with hydrogen from reaction 1 to become water. However, this disrupts the equilibrium and more H+ is produced to compensate.
