
Pages in this Section (10)
Amount of SubstanceThe Atom
Bonding
Chemical Equations
Electron Arrangement
Group 2
Intermolecular Forces
Mass Spectrometry
Periodicity
States and Shapes
Other Chemistry Sections
Applied Chemistry FundamentalInorganic
Organic
Physical Chemistry
Welcome to Fundamental Chemistry
The very basics of chemistry such as bonding and periodicity.
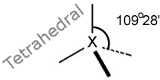 States and Shapes
States and Shapes
Molecules have different shapes, we look at the simple shapes of covalent molecules and the bond angles, including the well-known tetrahedral. States of matter, and different types of crystals. See States and Shapes
 Periodicity
Periodicity
Periodicity is to do with the patterns of behaviour and properties that are seen in the periodic table. How atomic radius, electronegativity and conductivity changes along period 3, and what the reasons for this are. See Periodicity
 Mass Spectrometry
Mass Spectrometry
A review of fundamental particles. The mass spectrometer is a useful tool to identify molecules, it has four steps: ionisation, acceleration, deflection and detection. How to interpret the graphs. See Mass Spectrometry
 Intermolecular Forces
Intermolecular Forces
Intermolecular bonding is a form of bonding between molecules. The weakest is Van der Waals followed by permanent dipoles and then the strongest: hydrogen bonding. These influence the boiling points of molecules. See Intermolecular Forces
 Group 2
Group 2
Group 2 is the alkaline earth metals, the trends in atomic radius, ionisation energy and electronegativity are examined. The reactions with water become more vigourous and they have varying solubilities. See Group 2
 Electron Arrangement
Electron Arrangement
The tricky subject of electron configuration, the various sub-shells and the notations used. The importance of ionisation energy and Hund's maximum multiplicity rule. The patterns across perioids and down groups. See Electron Arrangement
 Chemical Equations
Chemical Equations
Knowing how reactions are noted in chemistry is a vital first step. From word equations to using chemical symbols and adding state symbols and balancing. How equations can be used to calculate masses. See Chemical Equations
 Bonding
Bonding
Three types of bonding in substances: covalent, ionic and metallic, but these are not black and white and there is electronegativity to take in to account. We look at how the types of bonding affect properties. See Bonding
 The Atom
The Atom
An atom is the smallest unit of a particular substance that you can have. The numbers of protons, neutrons and electrons can be calculated using the mass number and proton number, all found in the periodic table. See The Atom
 Amount of Substance
Amount of Substance
The relative atomic mass and relative formula mass. What is a mole (in chemistry)? and how this relates to Avogadro's constant. The ideal gas equation links pressure, volume, moles, and gas constant and temperature. See Amount of Substance